Testicular seminoma
Updates to Article Attributes
Testicular seminomas are the most common testicular tumours and account for approximately 45~45% of all primary testicular tumours. This article concerns itself only with testicular seminomas - however, however, seminomas can arise outside of the testicle most often within the anterior mediastinum, e.g. anterior mediastinal germ cell tumours.
Epidemiology
Testicular germ cell tumours account for around 1-2% of all malignancies in males up to the age of 65, but they are the most common nonhematologic malignancy in males 15-49 years old. Approximately 50% of germ cell tumours are seminomas.
A higher frequency amongst Caucasians compared with African Americans has been observed (9:1)ref required.
Risk factors
-
undescended testis is the major risk factor for testicular germ cell tumours
- 10
-40 times-40x increased risk -
around 10~10% of tumours are associated with undescended testis - increased risk in the contralateral normally descended testis
- 10
- previous tumour in contralateral testis
- family history of testicular germ cell tumour
-
testicular microlithiasis: controversial risk factor, although some sources claim an
~8 x~8x increased risk 2 - other risk factors include infections such as HIV, mumps, orchitis
and, and history of trauma or organ transplant immunosuppression.
See article risk factors for testicular germ cell tumour for more detail.
Pathology
Seminoma by definition must be pure seminoma on histology and not associated with an elevated serum alpha fetoprotein (AFP). If either of these criteria is not met then the tumour must be classified as non-seminomatous and managed accordingly. Uncommonly (15%) b-HCG may be slightly elevated. Pure seminomas are subdivided into 3 histological sub types:
-
classic:85%, infrequent mitoses; monotonous sheet of large cells with abundant cytoplasm and round hyperchromatic nuclei, prominent nucleoli. -
anaplastic:10%, 3 or more mitotic figures per high-power field -
spermatocytic:5%, older male patients above 60 years old, rarely metastasise; well-differentiated with cells resembling secondary spermatids
On gross examination, seminomas are pale gray to yellow nodules that are uniform or slightly lobulated and often bulge from the cut surface. Tumour staging is via a TNM system (see testicular cancer staging).
Clinical presentation
The most common presentation is with a painless testicular mass although some 45% will report a degree of testicular discomfort. Bilateral tumours are rare (~2%) and are almost always asynchronous 1. Diagnosis following trauma is common as it draws the patient’s attention to the lump. Back pain, abdominal discomfort or abdominal mass may be a presenting feature in the 25% of patients who have retroperitoneal nodal metastases 3. Presentation with distant or extranodal metastases is rare (~5%).
Pathology
Seminoma by definition must be pure seminoma on histology and notassociated with an elevated serum alpha fetoprotein (AFP). If either of these criteria is not met then the tumour must be classified as non-seminomatous and managed accordingly. Uncommonly (15%) b-HCG may be slightly elevated. Pure seminomas are subdivided into three histological subtypes:
- classic: 85%, infrequent mitoses; monotonous sheet of large cells with abundant cytoplasm and round hyperchromatic nuclei, prominent nucleoli
- anaplastic: 10%, ≥3 mitotic figures per high-power field
- spermatocytic: 5%, older male patients above 60 years old, rarely metastasise; well-differentiated with cells resembling secondary spermatids
On gross examination, seminomas are pale gray to yellow nodules that are uniform or slightly lobulated and often bulge from the cut surface. Tumour staging is via a TNM system (see testicular cancer staging).
Radiographic features
Ultrasound
Ultrasound is the first line imaging modality if a patient presents with a testicular abnormality. Features include:
- seminomas usually appear as a homogeneous intratesticular mass of low echogenicity compared to normal testicular tissue
- the mass is usually oval and well-defined in the absence of local invasion
- usually confined within the tunica albuginea, rarely extending to paratesticular structures
- internal blood flow is seen on color Dopper imaging
- cystic regions and calcifications are less common than in non-seminomatous germ cell tumours
- larger seminomas can have a heterogenous appearance
CT
Abdominal and pelvic CT are important in visualizing metastases both as a part of primary staging seminoma but also in primary diagnosis when a testicular mass is unknown.
Metastases to the para-aortic lymph nodes at the level of the renal vessels are the typical first site of spread owing to the lymphatic drainage of the testicles relating to embryological testicular descent. The nodal metastases are often bulky, of homogenous density and tend to encase surrounding vessels.
Inguinal or iliac lymph node metastases suggest lymphatic spread via the scrotum and therefore local tumour extension beyond the tunica vaginalis.
Visceral metastases are seen in around 5~5% of patients at presentation (lung, liver, bone, brain). Staging CT of the chest is only indicated when regional para-aortic lymph node spread is present or if there is an abnormal CXRchest x-ray.
Following therapy, lymph node metastases reduce markedly in size but some inactive abnormal tissue persists which can be difficult to distinguish from residual disease and follow up monitoring is required.
MRI
Usually appear as multinodular tumours of uniform signal intensity 3-4.:
- T2: hypointense to normal testicular tissue
- T1+C (Gd): inhomogeneous enhancement
Nuclear medicine
-
FDG-PET
is less sensitive in initial staging than CT. Usually only used following treatment of seminoma to look for metabolic activity within residual lymphadenopathy or extranodal metastases.:
Treatment and prognosis
Treatment involves surgical removal of the testicular primary (orchiectomy).
Radiotherapy to regional nodes if there is local disease (stage I) or limited nodal para-aortic metastases (non-bulky stage II) may be necessary (seminomas are radiosensitive, whereas nonseminomatous tumours are not).
Chemotherapy may be necessary if there is bulky para-aortic lymph node involvement of more distant disease. Four cycles of bleomycin, etoposide, and cisplatin (BEP) is currently standard of care.
Prognosis is good for all stages with greater than 90% cure rate. Survivors have an increased life timelifetime risk of developing other malignancies including testicular cancer in the remaining testicle, mesothelioma and cancer of the lung, colon, bladder, pancreas, and stomach.
Differential diagnosis
The main differential for testicular mass in young adults is non-seminomatous germ cell tumour (NGCT) which usually appear more heterogenous, often with cysts and calcification. Lymphadenopathy of NGCT may enhance more heterogenously. Testicular lymphoma is the main differential diagnosis to consider when para-aortic lymphadenopathy is the presenting finding or in the setting of bilateral testicular lesions.
- see differential for unilateral testicular mass
- see differential for bilateral testicular masses
-<p><strong>Testicular seminomas</strong> are the most common <a href="/articles/testicular-cancer">testicular tumours</a> and account for approximately 45% of all primary testicular tumours. This article concerns itself only with testicular seminomas - however, seminomas can arise outside of the testicle most often within the <a href="/articles/anterior-mediastinum">anterior mediastinum</a>, e.g. <a href="/articles/anterior-mediastinal-germ-cell-tumours">anterior mediastinal germ cell tumours</a>. </p><h4>Epidemiology</h4><p>Testicular germ cell tumours account for around 1-2% of all malignancies in males up to the age of 65, but they are the most common nonhematologic malignancy in males 15-49 years old. Approximately 50% of germ cell tumours are seminomas. </p><p>A higher frequency amongst Caucasians compared with African Americans has been observed (9:1).</p><h5>Risk factors</h5><ul>- +<p><strong>Testicular seminomas</strong> are the most common <a href="/articles/testicular-cancer">testicular tumours</a> and account for ~45% of all primary testicular tumours. This article concerns itself only with testicular seminomas, however, seminomas can arise outside of the testicle most often within the <a href="/articles/anterior-mediastinum">anterior mediastinum</a>, e.g. <a href="/articles/anterior-mediastinal-germ-cell-tumours">anterior mediastinal germ cell tumours</a>. </p><h4>Epidemiology</h4><p>Testicular germ cell tumours account for around 1-2% of all malignancies in males up to the age of 65, but they are the most common nonhematologic malignancy in males 15-49 years old. Approximately 50% of germ cell tumours are seminomas. </p><p>A higher frequency amongst Caucasians compared with African Americans has been observed (9:1) <sup>ref required</sup>.</p><h5>Risk factors</h5><ul>
-<li>10-40 times increased risk </li>-<li>around 10% of tumours are associated with <a href="/articles/cryptorchidism">undescended testis</a>- +<li>10-40x increased risk </li>
- +<li>~10% of tumours are associated with <a href="/articles/cryptorchidism">undescended testis</a>
-<a href="/articles/testicular-microlithiasis">testicular microlithiasis</a>: controversial risk factor, although some sources claim an ~8 x increased risk <sup>2</sup>- +<a href="/articles/testicular-microlithiasis">testicular microlithiasis</a>: controversial risk factor, although some sources claim an ~8x increased risk <sup>2</sup>
-<li>other risk factors include infections such as HIV, mumps, orchitis and history of trauma or organ transplant immunosuppression. </li>-</ul><p>See article<a href="/articles/risk-factors-for-testicular-germ-cell-tumours"> risk factors for testicular germ cell tumour</a> for more detail.</p><h4>Pathology</h4><p>Seminoma by definition must be pure seminoma on histology and <strong>not </strong>associated with an elevated serum <a href="/articles/afp-elevation">alpha fetoprotein</a> (AFP). If either of these criteria is not met then the tumour must be classified as non-seminomatous and managed accordingly. Uncommonly (15%) b-HCG may be slightly elevated. Pure seminomas are subdivided into 3 histological sub types:</p><ul>- +<li>other risk factors include infections such as <a href="/articles/hivaids">HIV</a>, <a href="/articles/mumps">mumps</a>, <a href="/articles/orchitis">orchitis</a>, and history of <a href="/articles/testicular-trauma">trauma</a> or organ transplant immunosuppression</li>
- +</ul><p>See article<a href="/articles/risk-factors-for-testicular-germ-cell-tumours"> risk factors for testicular germ cell tumour</a> for more detail.</p><h4>Clinical presentation</h4><p>The most common presentation is with a painless testicular mass although some 45% will report a degree of testicular discomfort. Bilateral tumours are rare (~2%) and are almost always asynchronous <sup>1</sup>. Diagnosis following trauma is common as it draws the patient’s attention to the lump. Back pain, abdominal discomfort or abdominal mass may be a presenting feature in the 25% of patients who have retroperitoneal nodal metastases <sup>3</sup>. Presentation with distant or extranodal metastases is rare (~5%). </p><h4>Pathology</h4><p>Seminoma by definition must be pure seminoma on histology and not<strong> </strong>associated with an elevated serum <a href="/articles/afp-elevation">alpha fetoprotein</a> (AFP). If either of these criteria is not met then the tumour must be classified as non-seminomatous and managed accordingly. Uncommonly (15%) <a href="/articles/beta-hcg-levels">b-HCG</a> may be slightly elevated. Pure seminomas are subdivided into three histological subtypes:</p><ul>
-<strong>classic:</strong> 85%, infrequent mitoses; monotonous sheet of large cells with abundant cytoplasm and round hyperchromatic nuclei, prominent nucleoli.</li>- +<strong>classic:</strong> 85%, infrequent mitoses; monotonous sheet of large cells with abundant cytoplasm and round hyperchromatic nuclei, prominent nucleoli</li>
-<strong>anaplastic:</strong> 10%, 3 or more mitotic figures per high-power field</li>- +<strong>anaplastic:</strong> 10%, ≥3 mitotic figures per high-power field</li>
-</ul><p>On <a href="/cases/seminoma">gross examination</a>, seminomas are pale gray to yellow nodules that are uniform or slightly lobulated and often bulge from the cut surface. Tumour staging is via a TNM system (see <a href="/articles/testicular-cancer-staging">testicular cancer staging</a>). </p><h4>Clinical presentation</h4><p>The most common presentation is with a painless testicular mass although some 45% will report a degree of testicular discomfort. Bilateral tumours are rare (~2%) and are almost always asynchronous <sup>1</sup>. Diagnosis following trauma is common as it draws the patient’s attention to the lump. Back pain, abdominal discomfort or abdominal mass may be a presenting feature in the 25% of patients who have retroperitoneal nodal metastases <sup>3</sup>. Presentation with distant or extranodal metastases is rare (~5%). </p><h4>Radiographic features</h4><h5>Ultrasound</h5><p>Ultrasound is the first line imaging modality if a patient presents with a testicular abnormality. </p><ul>- +</ul><p>On <a href="/cases/seminoma">gross examination</a>, seminomas are pale gray to yellow nodules that are uniform or slightly lobulated and often bulge from the cut surface. Tumour staging is via a TNM system (see <a href="/articles/testicular-cancer-staging">testicular cancer staging</a>). </p><h4>Radiographic features</h4><h5>Ultrasound</h5><p>Ultrasound is the first line imaging modality if a patient presents with a testicular abnormality. Features include:</p><ul>
-</ul><h5>CT</h5><p>Abdominal and pelvic CT are important in visualizing metastases both as a part of primary staging seminoma but also in primary diagnosis when a testicular mass is unknown.</p><p>Metastases to the para-aortic lymph nodes at the level of the renal vessels are the typical first site of spread owing to the lymphatic drainage of the testicles relating to embryological testicular descent. The nodal metastases are often bulky, of homogenous density and tend to encase surrounding vessels.</p><p>Inguinal or iliac lymph node metastases suggest lymphatic spread via the scrotum and therefore local tumour extension beyond the tunica vaginalis.</p><p>Visceral metastases are seen in around 5% of patients at presentation (lung, liver, bone, brain). Staging CT of the chest is only indicated when regional para-aortic lymph node spread is present or if there is an abnormal CXR.</p><p>Following therapy, lymph node metastases reduce markedly in size but some inactive abnormal tissue persists which can be difficult to distinguish from residual disease and follow up monitoring is required.</p><h5>MRI</h5><p>Usually appear as multinodular tumours of uniform signal intensity <sup>3-4</sup>.</p><ul>- +</ul><h5>CT</h5><p>Abdominal and pelvic CT are important in visualizing metastases both as a part of primary staging seminoma but also in primary diagnosis when a testicular mass is unknown.</p><p>Metastases to the para-aortic lymph nodes at the level of the renal vessels are the typical first site of spread owing to the lymphatic drainage of the testicles relating to embryological testicular descent. The nodal metastases are often bulky, of homogenous density and tend to encase surrounding vessels.</p><p>Inguinal or iliac lymph node metastases suggest lymphatic spread via the scrotum and therefore local tumour extension beyond the tunica vaginalis.</p><p>Visceral metastases are seen in ~5% of patients at presentation (lung, liver, bone, brain). Staging CT of the chest is only indicated when regional para-aortic lymph node spread is present or if there is an abnormal chest x-ray.</p><p>Following therapy, lymph node metastases reduce markedly in size but some inactive abnormal tissue persists which can be difficult to distinguish from residual disease and follow up monitoring is required.</p><h5>MRI</h5><p>Usually appear as multinodular tumours of uniform signal intensity <sup>3-4</sup>:</p><ul>
-</ul><h5>Nuclear medicine</h5><ul><li>-<strong>FDG-PET:</strong> less sensitive in initial staging than CT. Usually only used following treatment of seminoma to look for metabolic activity within residual lymphadenopathy or extranodal metastases.</li></ul><h4>Treatment and prognosis</h4><p>Treatment involves surgical removal of the testicular primary (orchiectomy).</p><p>Radiotherapy to regional nodes if there is local disease (stage I) or limited nodal para-aortic metastases (non-bulky stage II) may be necessary (seminomas are radiosensitive, whereas nonseminomatous tumours are not).</p><p>Chemotherapy may be necessary if there is bulky para-aortic lymph node involvement of more distant disease. Four cycles of bleomycin, etoposide, and cisplatin (BEP) is currently standard of care.</p><p>Prognosis is good for all stages with greater than 90% cure rate. Survivors have an increased life time risk of developing other malignancies including testicular cancer in the remaining testicle, mesothelioma and cancer of the lung, colon, bladder, pancreas, and stomach. </p><h4>Differential diagnosis</h4><p>The main differential for testicular mass in young adults is <a href="/articles/non-seminomatous-germ-cell-tumours-2">non-seminomatous germ cell tumour</a> (NGCT) which usually appear more heterogenous, often with cysts and calcification. Lymphadenopathy of NGCT may enhance more heterogenously. <a href="/articles/testicular-lymphoma">Testicular lymphoma</a> is the main differential diagnosis to consider when para-aortic lymphadenopathy is the presenting finding or in the setting of bilateral testicular lesions.</p><ul>- +</ul><h5>Nuclear medicine</h5><p>FDG-PET is less sensitive in initial staging than CT. Usually only used following treatment of seminoma to look for metabolic activity within residual lymphadenopathy or extranodal metastases.</p><h4>Treatment and prognosis</h4><p>Treatment involves surgical removal of the testicular primary (orchiectomy).</p><p>Radiotherapy to regional nodes if there is local disease (stage I) or limited nodal para-aortic metastases (non-bulky stage II) may be necessary (seminomas are radiosensitive, whereas nonseminomatous tumours are not).</p><p>Chemotherapy may be necessary if there is bulky para-aortic lymph node involvement of more distant disease. Four cycles of bleomycin, etoposide, and cisplatin (BEP) is currently standard of care.</p><p>Prognosis is good for all stages with greater than 90% cure rate. Survivors have an increased lifetime risk of developing other malignancies including testicular cancer in the remaining testicle, mesothelioma and cancer of the lung, colon, bladder, pancreas, and stomach. </p><h4>Differential diagnosis</h4><p>The main differential for testicular mass in young adults is <a href="/articles/non-seminomatous-germ-cell-tumours-2">non-seminomatous germ cell tumour</a> (NGCT) which usually appear more heterogenous, often with cysts and calcification. Lymphadenopathy of NGCT may enhance more heterogenously. <a href="/articles/testicular-lymphoma">Testicular lymphoma</a> is the main differential diagnosis to consider when para-aortic lymphadenopathy is the presenting finding or in the setting of bilateral testicular lesions.</p><ul>
Image 3 Ultrasound ( create )

Image 4 Ultrasound (Longitudinal) ( update )
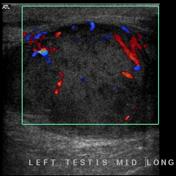
Image 5 CT (C+ portal venous phase) ( update )

Image 8 MRI (T2 fat sat) ( update )








 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.